Abstract
Carbon monoxide (CO) at low, non-toxic concentrations has recently emerged as a powerful biological response modifier that exerts anti-inflammatory protection in many animal models of tissue inflammation and injury including sickle cell disease (SCD). The protection observed was associated with modulation of the innate and adaptive immune response and restoration of tissue and organ function. CO may also have a role as an antipolymerization hemoglobin S agent. Inhaled CO gas has been used to generate the majority of preclinical and clinical data regarding CO. However, inhaled CO is challenging to precisely dose given the variability of patient ventilation. Furthermore inhaled CO has environmental safety concerns for patients, as it requires the presence of large amounts of compressed CO gas in cylinders. Oral administration of CO avoids many of the issues associated with gas administration and provides a platform for outpatient administration. An oral formulation that would deliver low, non-toxic concentrations of CO, might be a better alternative for treating SCD patients.
To test the efficacy of oral administration of CO, a liquid CO formulation (HBI-002) was administered by gavage to NY1DD and Townes-SS transgenic mouse models of SCD. Blood CO-hemoglobin (COHb) levels peaked within 5 minutes of oral administration. Baseline COHb levels were 1.6% and 1.8% in vehicle-treated NY1DD and Townes-SS sickle mice, respectively, compared to 0.9% in untreated Townes AA control mice (p<.05). Five minutes after oral gavage with HBI-002 (10 ml/kg, ~0.2 ml/mouse), COHb levels peaked at 5.4% in NY1DD mice and 4.7% in Townes-SS mice (p<.05 compared to vehicle).
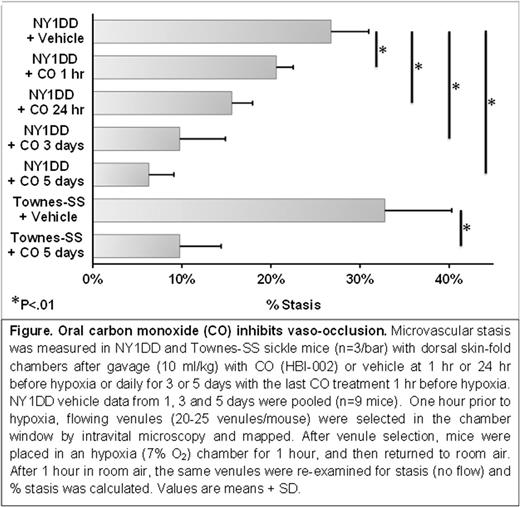
Oral CO (10 ml/kg) administered once daily for 1, 3 or 5 days inhibited hypoxia-induced microvascular stasis in NY1DD SCD mice in a duration dependent manner. Stasis was 27% in vehicle-treated mice, 21% when oral CO was administered 1 hour before hypoxia and 16% when administered 24 hours before hypoxia. Stasis was 10% after 3 daily treatments, and 6% after 5 daily treatments (Figure). In Townes-SS mice, stasis was 33% in vehicle-treated mice and 10% after 5 daily CO treatments (p<.01, CO versus vehicle).
The effects of oral CO on blood counts were examined in Townes-SS mice treated with oral CO (n=4) or vehicle (n=3) (10 ml/kg, once daily X 10 days). Hemoglobin levels rose from 5.3 g/dL in vehicle-treated mice to 6.3 g/dL in CO-treated mice (p<.05). Similarly, red blood cell counts rose from 2.36 X 106/µL in vehicle-treated mice to 2.89 X 106/µL in CO-treated mice (p<.05). In concordance with these findings, hematocrits rose from 26.3% in vehicle-treated mice to 30.0% in CO-treated mice (p<.05). The percent reticuloctyes was not significantly different: 48.4% in vehicle-treated mice and 45.0% in CO-treated mice. The percentage of stained F-cells was similar (16%) in both treatment groups. White blood cell counts decreased from 29.1 X 103/µL in vehicle-treated mice to 20.3 X 103/µL in CO-treated mice (p<.01). Hepatic expression of two key anti-inflammatory proteins, Nrf2 and heme oxygenase-1 (HO-1), was increased in CO-treated SS mice. In agreement with these findings, hepatic expression of two pro-inflammatory proteins, NF-κB phospho-p65 and VCAM-1, was decreased in CO-treated SS mice.
These data suggest that once daily oral CO treatment, at COHb levels below known toxicity, may have therapeutic potential in SCD. Further research is needed to carefully elucidate the safety and benefits of this potential gasotransmitter therapy in SCD patients who have many pathophysiological complications of this devastating disease.
Belcher: Hillhurst Biopharmaceuticals, Inc.: Other: I have an SBIR grant from NHLBI with Hillhurst Biopharmaceuticals, Inc.. Gomperts: Hillhurst Biopharmaceuticals, Inc.: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Levy: Hillhurst Biopharmaceuticals, Inc.: Consultancy, Other: Senior Medical Advisor. Otterbein: Hillhurst Biopharmaceuticals, Inc.: Consultancy, Other: Principal Scientific Advisor. Vercellotti: Hillhurst Biopharmaceuticals, Inc.: Other: I have an SBIR grant with Hillhurst Biopharmaceuticals, Inc.
Author notes
Asterisk with author names denotes non-ASH members.